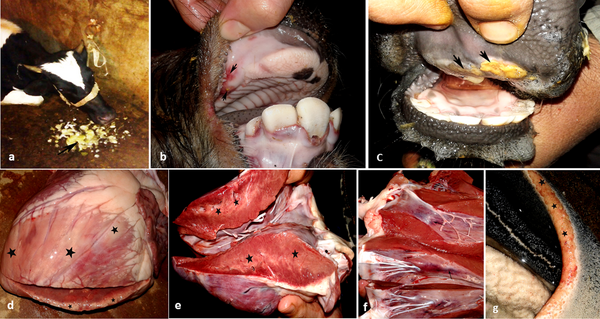
If a case of foot and mouth disease (FMD) is confirmed
anywhere in the United States, it could spread rapidly across
the nation. If any animal on your farm is confi rmed to have
FMD, all animals on the farm that could get sick (cattle, swine,
sheep and goats) may be euthanized and disposed of to
control the further spread of the disease.
There are steps you can take to help prevent FMD from
entering your farm. Strict biosecurity practices can help you
and your neighbors minimize the chances that your animals
will have to be destroyed.
A General Precautions handout (found on the CFSPH website)
provides prevention steps that should always be used on a
farm.
The biosecurity practices outlined here should be put into
place immediately if FMD is confi rmed anywhere in the U.S.
and maintained until the U.S. is once again declared FMD free.
Restrictions will depend on the
scope of the outbreak.
- These measures will minimize the spread of
FMD to other areas, including your farm. - Post signs at the farm entrance to inform visitors of procedures to follow on your farm.
- Stay off this farm unless given permission to enter.
- Honk before getting out of vehicle (to announce your arrival).
- Check-in with farm personnel upon arrival. (Direct visitor to “where” they should check-in).
- Follow farm biosecurity procedures.
- Wear protective clothing (coveralls, boots)
while on the farm. (Be sure to guide visitors to where protective clothing is located). - Visitors and their vehicles should avoid contact
with animals, unless absolutely necessary. - Traffi c on or off your farm should be closely
monitored and recorded. (See Appendix B) - Maintain a log sheet to record all visitors and vehicles that enter your farm.
- All visitors should be accompanied by someone from your farm at all times.
- Accurate record keeping of traffi c on your
farm will help with disease surveillance and
tracking should it become necessary. - Do not rely on your ability to “recall” visitors and vehicles that were on your farm.
General Precautionary Measures
Prevention measures to minimize the introduction and spread
of FMD onto your farm fall into three general categories.
Use strict biosecurity measures for animals, animal products, vehicles, people and equipment.
Restrict or stop all animal movement to prevent entry or spread of the disease.
Observe, detect and report any disease or unusual signs to your herd veterinarian as quickly as possible.
Specifi c steps you can take upon FMD being confi rmed in
the United States are listed below. Many should already be in
place on your farm but should be enhanced and more strictly
enforced in the event that FMD is confi rmed in the U.S. This
will minimize the chance of the disease being introduced onto
your farm.
Farm Entrance
- Limit access to your farm.
- The entrance to your farm is a major control point.
- Have only one gated entrance to the animal areas on your farm to better control and monitor
all visitors and vehicles arriving at your farm. - Keep the gate locked when not in use.
- Stop all movement of animals on and off your farm.
- If FMD is confi rmed in the U.S., movement restrictions may be implemented locally, regionally and possibly nationally.
PREVENTION PRACTICES
- Do not rely on your ability to “recall” visitors
and vehicles that have accessed your farm. - Restrict areas where vehicle traffi c can go.
- Establish parking areas away from any animals, barns and livestock areas, preferably on concrete or paved areas.
- Avoid vehicle transfer of dirt, mud or manure.
- Stop all movement of animals on and off farm.
- In the event of FMD in the U.S., movement restrictions will likely be ordered.
- This serves to not only minimize the spread of FMD
but may prevent it from getting onto your farm. - If you are allowed to move animals, clean
and disinfect the vehicle and trailer before loading and after unloading. - Pay special attention to the tires and wheel wells.
- Do not mix livestock species in vehicles when transporting animals.
- Monitor animals closely and frequently for
any developing illness or signs of disease. - Isolate sick animals from the herd
to minimize disease spread. - Isolation should be at a minimum 28-30 days
(which equals two incubation periods for FMD). - Contact your herd veterinarian immediately to examine sick animals.
- Use separate facilities, equipment and
staff to handle isolated livestock. - If this is not possible, at a minimum, handle or visit the isolated animals LAST.
- Clean and disinfect all equipment, clothing, boots,
etc. that come into contact with ill animals. - Do not acquire semen from FMD-outbreak areas.
- Semen can contain large amounts of the FMD virus.
- Any animals that have recently been purchased or have returned to the farm
should be quarantined for 30 days. - New or returning animals (e.g. shows,
competitions) can be infected with a disease without showing signs right away. - Quarantine allows time for a disease to develop in
the animal, without exposing your entire herd to
the disease agent. The animal can then be examined, diagnosed and treated (if it is not FMD). - Animals exposed to the FMD virus can take as
long as 14 days before signs of illness are seen. - Do not allow new additions and animals returning to share water, feed, facilities or bedding with your other animals.
- Ideally, animals should be quarantined
at a separate location (premises).
Animals
Livestock
- Do not allow contact of your animals with neighbor’s livestock.
- Move animals out of pastures or lots where
they have contact with neighboring animals. - Provide as much distance between your animals and neighboring animals as possible.
- Consider double fencing the perimeters
to minimize nose-to-nose contact. - Fence off streams and rivers.
- Provide fresh drinking water in tanks.
Wildlife and Other Animals
- Prevent contact with free roaming animals (wildlife, cats, dogs).
- Free roaming animals can potentially spread the
FMD virus from infected to susceptible animals. - Keep pets in a kennel or tied securely to avoid
contact with livestock and feed areas. - Ask your neighbors to do the same.
Cleaning and Disinfection
General
- Remove any organic material before any cleaning or disinfection.
- Most disinfectants are ineffective when dirt,
manure and other debris are present. - This step applies to boots and vehicles
as well as buildings being cleaned.
Boot Baths
- Anyone allowed in animal areas should be required to wash and disinfect their boots prior
to entering and when exiting animal areas. - Maintain boot baths properly.
- Mix solution to the proper concentration according to the label instructions.
- Clean all dirt, manure and debris off of boots
BEFORE stepping into the disinfectant solution. Organic material (dirt, manure, etc) will
prevent most disinfectants from working. - Allow the disinfectant solution to have ample contact time with the boot surface. This will vary with
the disinfectant selected. Consult the product label. - Change solutions daily or when visibly dirty
FAQ’s
What are the key biosecurity measures to prevent the spread of Foot and Mouth Disease (FMD)?
Answer:
Key biosecurity measures include restricting the movement of animals, disinfecting vehicles, equipment, and footwear, isolating new or sick animals, and controlling the access of visitors to farms.
2. How does vaccination help in preventing Foot and Mouth Disease (FMD)?
Answer:
Vaccination boosts the immunity of animals against FMD, reducing the risk of infection and limiting the spread of the disease within and between farms.
3. Why is early detection important in the prevention of Foot and Mouth Disease (FMD)?
Answer:
Early detection allows for immediate action, such as isolating infected animals, notifying authorities, and implementing control measures to prevent widespread outbreaks.
Need Veterinary Help?
If you have questions about animal health, management, diseases, or treatment, our professional veterinary team is here to assist you.
📞 Consult Online: Contact Us
📧 Email: professionaldvm129@gmail.com
🌐 Website: www.veterinaryhub.info
Disclaimer: The information provided in this article is for educational purposes only. Always consult a licensed veterinarian for accurate diagnosis and treatment.